Most of us will probably be able to recall at least vaguely that a molecule called ATP is essential for making our bodies move, but this molecule is only a small part of a much larger system. Although we usually aren’t aware of it, our bodies consist of a massive collection of biological motors and related structures, which enable our muscles to contract, nutrients and fluids to move around, and our cells to divide and prosper. Within the biochemical soup that makes up single- and multi-cellular lifeforms, it are these mechanisms that turn a gooey soup into something that can do much more than just gently slosh around in primordial puddles.
There are many similarities between a single-cell organism like a bacteria and eukaryotic multi-cellular organisms like us humans, but the transition to the latter requires significantly more complicated structures. An example for this are cilia, which together with motor proteins like myosin and kinesin form the foundations of our body’s basic functioning. Quite literally supporting all this is the cytoskeleton, which is a feature that our eukaryotic cells have in common with bacteria and archaea, except that eukaryotic cytoskeletons are significantly more complex.
The Cytoskeleton
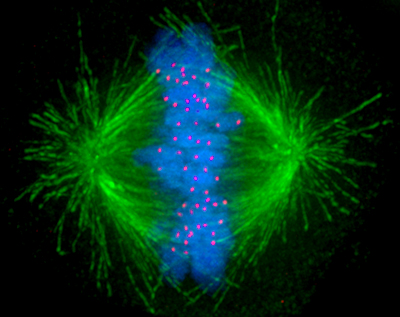
We mammals have a skeleton to keep our bodies from collapsing into a sad, soggy pile, so too do our cells have their own skeleton, giving them shape and rigidity, as well as providing motor proteins something to interact with. The cytoskeleton in eukaryotes consists of mainly microfilaments, intermediate filaments and microtubules, with prokaryotes having their own distinct cytoskeleton structures. Of the three types that make up the eukaryotic cytoskeleton, the microfilament and microtubules are used by motor proteins. These thus fall into two categories: actin motors (using the actin-based microfilaments) and microtubule motors.
Although muscles are an obvious example of motor proteins in action, even something as fundamental as cell division (mitosis) involves motor proteins, specifically kinesin microtubule motors. Starting from a centrosome (microtubule organizing center), microtubules are formed from tubulin to create the scaffolding for the kinesin proteins to move across, which then move the two centrosomes (one newly formed) to opposite sides of the cell undergoing mitosis. What drives the actual separation of the duplicated chromosomes (chromatids) are the kinetochores. These kinetochore proteins are microtubule-binding structures that form not only the linkage between the chromatids and a centromere, they also create the mitotic spindle, and which use ATP to ‘crawl’ along the microtubules thanks to their microtubule-binding dynein and kinesin motor proteins. This is what pulls the chromatids apart, allowing mitosis to continue and eventually end up with two sets of DNA within one cell.
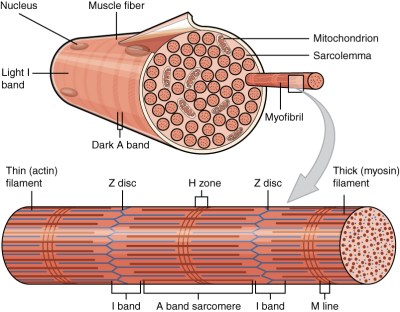
The motor proteins that create muscle cells do not use microtubules, but rather the actin-based microfilaments. Within mammalian species, there are about 40 different types of these myosin motor proteins. The protein myosin II is the one that is part of muscle cells, but it also serves an essential function with mitosis, specifically after the completion of mitosis, when the cytokinesis stage commences. During this an actin-myosin ring is assembled around the cell, along which myosin II proteins can move. Powered by ATP, these motor proteins constrict the cell, pinching it until one cell becomes two, each with its own copy of the original cell’s DNA.
In the case of muscle cells, these are rather unique in this regard, as some of them are multinucleated cells, formed through the fusion of individual cells (a synctium). Mammalian muscle tissues come in three broad categories: smooth, cardiac and skeletal muscle tissue, each of which have distinct properties. Of these skeletal muscle tissue is composed of synctium cells, which form long tubular cells, inside of which are many myofibril organelles. These myofibrils consist of myofilaments, each of which can be a thick, thin or elastic type.
The thick filaments are myosin II proteins, the thin filaments are actin proteins and the elastic filaments (titin-based), which provides support and guidance to the thick and thin filaments. Muscle contraction is thus accomplished by the myosin II filaments binding to the actin and moving across it, powered by the ATP from the mitochondria (the powerhouses of the cell). This shortens the myofibril and thus the muscle. Relaxation of the muscle involves the enzyme acetylcholinesterase, which breaks down the neurotransmitter acetylcholine, which originally excited the muscle fiber membrane’s receptors.
Protein Power
Often referred to as ‘cellular currency’, ATP (adenosine triphosphate) and GTP (guanosine triphosphate) are both nucleoside triphosphates, which are an essential precursor to RNA and DNA in addition to being involved in signaling pathways, and the aforementioned energy currency. These nucleosides are generally synthesized inside cells, in the case of ATP using mechanisms like photosynthesis and cellular respiration. While ATP is the most important energy carrier within the cell, GTP is important in DNA transcription and microtubule polymerization, making its use more specialized.
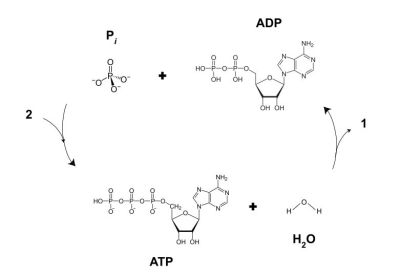
The energy of ATP and GDP is released through hydrolysis, which produces ADP and GDP, respectively, along with a free inorganic phosphate ion (Pi). This releases about 20.5 kilojoules per mole, with the hydrolyzed molecules being ‘recharged’ to produce new ATP and GTP, in a continuous cycle.
For hydrolysis of ATP, the enzyme ATPase has to be present. In the case of e.g. muscle tissue, the ATP will bind to the myosin II proteins, which subsequently gets hydrolyzed by the ATPase, turning it into ADP and Pi. This process forms cross-bridges between the myosin II and actin, which induces movement of the former along the latter, and releasing the ADP and Pi. This is followed by a fresh ATP binding to the myosin II, preparing it for the next power stroke. For different motor proteins a similar process enables a similar process of events, which can continue for as long as it is mechanically possible, fresh ATP (or GTP) is available and an impetus (e.g. neurotransmitter binding to a receptor) is present.
Cilia
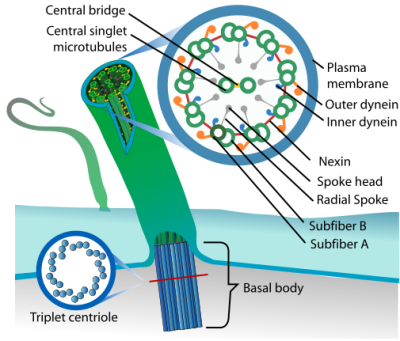
Perhaps one of the most fascinating motor proteins are those that are part of flagella and cilia. Here the bacterial flagellum is quite different from the eukaryotic one, being powered by a proton gradient motor, and also different from the archaeal flagella. Meanwhile the eukaryotic flagella and cilia are quite similar, with the distinction being mostly academic. Both consist of nine microtubules with a pair of dynein motor proteins per doublet microtubule that use ATP hydrolysis to provide motion. The only exception here are the non-motile cilia, which lack the dynein.
Dynein motor proteins move along microtubules, which makes their presence in these flagella and cilia rather logical. These motile cilia and flagella are found throughout the body, with the respiratory epithelial cells found throughout the inside of the respiratory tract providing the essential function of mucociliary clearance, and similar motile cilia moving cerebrospinal fluid inside the brain, as well as egg cells from the oviducts (fallopian tubes) to the uterus .
Meanwhile the version found on sperm cells which provide them with the ability to propel themselves are generally called flagella. This version is longer and has a different undulating motion than the motile cilia described earlier, but still has the same basic structure. As said earlier, eukaryotic flagella and cilia are effectively the same, which has led to considerable confusion and debate in the past.
A Wonder Of Evolution
In this article we touched only upon a fraction of the sheer complexity of all the details which make a body like that of ours work (somewhat) perfectly on a daily basis. Beyond the essentials covered on e.g. Wikipedia, there are the in-depth reference books, with the student reference work Biochemistry (8th edition Archive link) by Jeremy M. Berg and colleagues my current go-to refresher on just about anything to do with biochemical systems.
It should come as no surprise that with the sheer complexity of the field of biochemistry, even something as relatively straightforward as motor proteins would lead to significant confusion. This was quite obvious in a recent video on the Smarter Every Day YouTube channel, where the differences between bacterial and eukaryotic flagella got mixed up severely, which was perhaps somewhat ironic for a science channel that is run by a person with rather strong opinions on ‘intelligent design’ (ID).
The complexity of biological motors is often pointed to by ID proponents as some kind of evidence of ‘irreducible complexity’, yet across the bacterial, archaeal and eukaryotic domains we can see the same problems being solved repeatedly in three very distinct fashions. This shows quite clearly the marvel of evolution, and how this process over millions of years can turn even the most complex problem into a logical series of steps once you get the right chemicals together.
Once the chemistry had some time to turn into proper biochemistry with the evolutionary survival process mercilessly picking off the attempts that weren’t quite good enough, and before you know it you have us primates marveling at at said biochemistry. As they say, life finds a way.
Featured image: “Flagellar Motor Assembly” by [PKS615].