A team at MIT led by [Professor Douglas Hart] has discovered a new, potentially revelatory method for the generation of hydrogen. Using seawater, pure aluminum, and components from coffee grounds, the team was able to generate hydrogen at a not insignificant rate, getting the vast majority of the theoretical yield of hydrogen from the seawater/aluminum mixture. Though the process does use indium and gallium, rare and expensive materials, the process is so far able to recover 90% of the indium-gallium used which can then be recycled into the next batch. Aluminum holds twice as much energy as diesel, and 40x that of Li-Ion batteries. So finding a way to harness that energy could have a huge impact on the amount of fossil fuels burned by humans!
Pure, unoxidized aluminum reacts directly with water to create hydrogen, as well as aluminum oxyhydroxide and aluminum hydroxide. However, any aluminum that has had contact with atmospheric air immediately gets a coating of hard, unreactive aluminum oxide, which does not react in the same way. Another issue is that seawater significantly slows the reaction with pure aluminum. The researchers found that the indium-gallium mix was able to not only allow the reaction to proceed by creating an interface for the water and pure aluminum to react but also coating the aluminum pellets to prevent further oxidization. This worked well, but the resulting reaction was very slow.
Apparently “on a lark” they added coffee grounds. Caffeine had already been known to act as a chelating agent for both aluminum and gallium, and the addition of coffee grounds increased the reaction rate by a huge margin, to the point where it matched the reaction rate of pure aluminum in deionized, pure water. Even with wildly varying concentrations of caffeine, the reaction rate stayed high, and the researchers wanted to find out specifically which part of the caffeine molecule was responsible. It turned out to be imidazole, which is a readily available organic compound. The issue was balancing the amount of caffeine or imidazole added versus the gallium-indium recovery rate — too much caffeine or imidazole would drastically reduce the recoverable amount of gallium-indium.
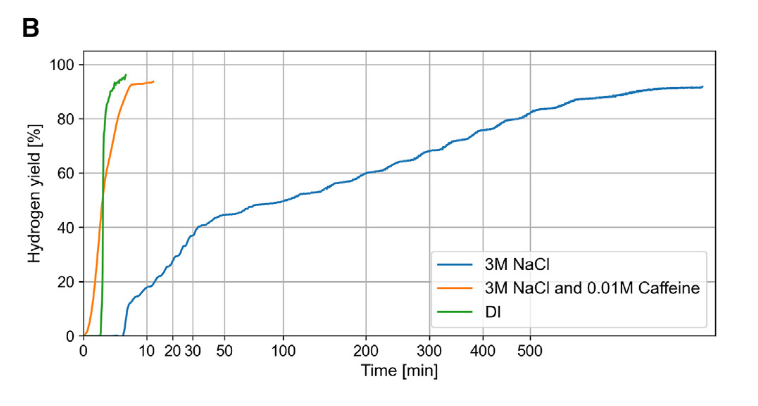
After some experimentation, they hit a magic number: a 0.02M concentration of imidazole resulted in consistent recovery rates of ~90% of the gallium-indium, which is comparable to the recovery rate in seawater with no catalysts of any kind! This method of hydrogen generation could make marine applications of hydrogen engines much more viable. By only needing to carry aluminum, imidazole and gallium-indium, the safety issues with liquid or compressed hydrogen disappear. This could make marine vehicles cleaner and more efficient while reducing the safety issues already present in carrying diesel or other marine fuels aboard.
The study goes into much, much more detail, so if you want to learn more, be sure to check it out! Thankfully, it’s hosted in an open-access journal so the knowledge is free for all to learn from.
[Thanks to zoobab for the tip, via ScienceDaily!]
Header image CC-BY-SA 4.0
